Understanding the pathogenic agent is crucial to understanding any disease; Echinococcosis is no exception. Echinococcosis’s impact as an infectious disease is centered around the actual helminths that cause the disease. And because there are two main forms of Echinococcosis with two different agents — Alveolar and Cystic Echinococcosis — the study of the agent becomes even more crucial.
The global study of Echinococcosis creates a database of knowledge that allows for researchers to understand how the disease varies across the world and what this means for future treatment and prevention. As Rojas, Romig and Lightowlers detail in their 2013 International Journal For Parasitology article entitled “Echinococcus granulosus sensu lato genotypes infecting humans – review of current knowledge,” the different forms of Echinococcosis pathogens call for different responses and treatments. They review the global understanding of the variance in the pathogen, and attempt to discern what this knowledge means for Echinococcosis treatment and prevention going forward.
Cystic Echinococcosis (CE), or hydatid disease, is caused by the larval form of the Echinococcus granulosus tapeworm. As a neglected tropical disease, CE largely affects poorer rural areas with large populations of dogs and sheep. However, not all forms of CE are identical. Through looking at nuclear DNA to understand rNA-encoding regions, different researchers across the world were able to divide the Echinococcus granulosus species into smaller groups (Rojas, Romig and Lightowlers, 2013). Researchers continued to analyze E. granulosus genes using mitochondrial genes, with the most important work differentiating the different species of E. granulosus coming from the work of Josephine Bowles using the cox1 and nad1 genes (Josephine Bowles et al., 1992).
The pathogen can be further classified into ten further genotypes, each genetically unique and given a label (G1-10). Rojas, Romig and Lightowlers (2013) break up the ten genotypes into three main four main groups: Echinococcus granulosus sensu stricto, Echinococcus equinus, Echinococcus ortleppi, and Echinococcus canadensis. Each group has myriad characteristics and differences, from their hosts to the location of the specific form of CE they cause.
G1-3: Echinococcus granulosus sensu stricto
Consisting of three different genotypes, the Echinococcus granulosus sensu stricto genotypic cluster contains far and away the most impactful forms of CE. The G1 genotype alone is responsible for 88.44% of human infections worldwide, and is distributed around the world. Studying the geographical differences among these different genotypes has led to hypothesizing that the Echinococcus granulosus sensu stricto originates in the Middle East and has subsequently spread to other areas. Within its wide range of infective areas — it is present in every continent but Antarctica — certain pockets with high CE prevalence exist where G1 is the only cause of human CE. Its geographical ubiquity can be attributed to the ubiquity of the intermediate hosts associated with Echinococcus granulosus sensu stricto: namely sheep, cattle and water buffalo. These genotypes are also often associated with cosmopolitan distribution, travelling large distances and spanning larger areas.
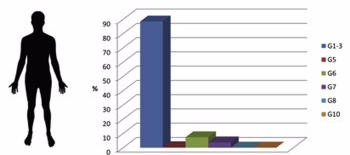
As shown in the chart, genotypes G1-3 (blue) chiefly contribute to human infection.
The important note about the Echinococcus granulosus sensu stricto genotypes is the presence of a vaccine. While these diseases continue to affect the large majority of victims of CE, there is a potential solution. The EG95 protective vaccine antigen has been developed to treat the G1 form of CE specifically. Distributing this vaccine is another matter entirely, but through examining the other genotypes researchers hope to determine whether the EG95 vaccine can protect against multiple forms of CE.
G4: Echinococcus equinus
Only a single genotype, the Echinococcus equinus helminths are most closely associated with its presence in species of the Equidae taxonomic family. Although closely related to the other genotypes of Echinococcus granulosus, Echinococcus equinus has actually never been known to infect humans with CE. This distinction has since been noted in its taxonomic name, as the genotype was initially referred to as Echinococcus granulosus equinus but has since become Echinococcus equinus. It is, however, a specific parasite of Equidae such as horses, donkeys and zebras; Echinococcus equinus is a horse-adapted species that is still present across the world. Recently this G4 genotype was found for the first time in a primate, as Boufana et al. (2012). describe Echinococcus equinus being found in a lemur.
G5: Echinococcus ortleppi
Again a subdivision of E. granulosus consisting of only a single genotype, Echinococcus ortleppi are also not particularly responsible for large-scale human infection of CE. Infections by the G5 genotype have been dubbed “rare” but their similarities to other CE-causing pathogens cannot be overlooked. Echinococcus ortleppi are the adult worm forms of the cattle-associated E. granulosus, and originally adapted to cattle as its intermediate hosts. While the helminth is now extinct in central Europe (its supposed place of origin) it has found hosts in animals across the world. Pigs in Africa, India and South America all carry the G5 genotype. Of more concern to human populations is the recent, albeit slight, rise of G5 in Vietnamese monkeys.
G6-10: Echinococcus canadensis
The largest genotypic cluster of E. granulosus, genotypes G6-10 are all referred to as Echinococcus canadensis. Within this cluster, the existence of the G9 genotype has yet to be fully validated and the G8 and G10 genotypes have only caused cases of Cystic Echinococcosis very rarely. However the G6 and G7 genotypes pose a significantly larger problem for human CE infection. Combined they are responsible for 11.07% of human infections worldwide — with G6 constituting 7.34% and G7 the other 3.73%. The diversity of the Echinococcus canadensis cluster poses challenges for its study and treatment. While only four main genotypes are identified, numerous variants and intermediate forms of Echinococcus canadensis exist. For instance, the G6 genotype exists mainly in Africa and Asia where it is transmitted by camels and goats, and in South America where it is transmitted only by goats; the G7 genotype is mainly existent in Eastern European pigs.
Takeaways & Conclusion
While Rojas, Romig and Lightowlers call attention to the specifics of the varying genotypes of Echinococcus granulosus in “Echinococcus granulosus sensu lato genotypes infecting humans – review of current knowledge” (2013), they end the review with a brief summation of how the different genotypes will influence the future of CE research. The EG95 protective vaccine is their main hope for the prevention of CE, and they determine the vaccine should be trialled to determine its effectiveness for the G6 and G7 genotypes (as well as the G1 genotype it was designed to treat). The variance of the different forms of Echinococcus granulosus contributes to Echinococcosis’s status as a neglected tropical disease, and a furthered understanding of the different agents will lead to advancements and more significant treatments of the disease in the areas that need them most.
The entire article can be found here.
Rojas, Cristian A. Alvarez, Thomas Romig, and Marshall W. Lightowlers. “Echinococcus granulosus sensu lato genotypes infecting humans–review of current knowledge.” International Journal for Parasitology 44.1 (2014): 9-18.